Clinical Applications: Lupus (SLE)
NucleoCapture® for Systemic Lupus Erythematosus (SLE)
NucleoCapture® mode of action
SLE is an autoimmune disease caused by breaking the immune tolerance against self , leading to uncontrolled immune response to autoantigens. Neutrophil Extracellular Traps (NETs) and other chromatin associated molecular patterns are major source of lupus autoantigens contributing to the breakdown of self-tolerance, immune complex formation and inflammation in SLE.
NucleoCapture® therapeutic apheresis session results in a rapid and sustained reduction of concentration NETs and other chromatin associated molecular patterns within the circulation. All existing or evolving therapeutic options in SLE are focused on the immunosuppression of the ongoing autoimmune response. In contrast, NucleoCapture is a first in class treatment providing selective removal of primary lupus autoantigens without immunosuppression.
In August 2025, the U.S. FDA granted Breakthrough Device Designation for NucleoCapture® for the treatment of severe SLE recognizing the urgent medical need and first-in-class novel mechanism of action.
Clinical development
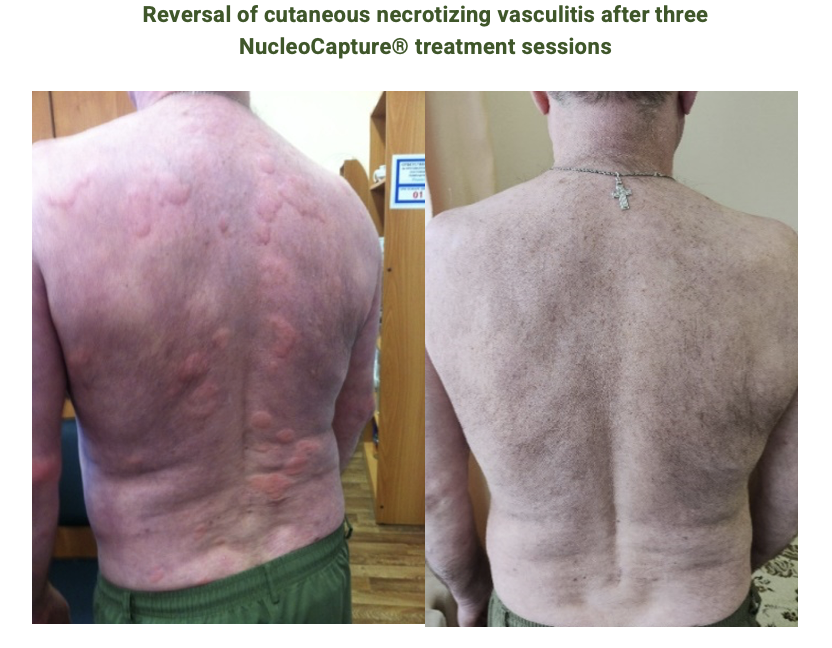
Clinical benefit from cfDNA plasma adsorption with NucleoCapture® for SLE patients has been confirmed in a series of clinical cases involving compassionate use of NucleoCapture® in patients with severe SLE flares. All treated patients showed rapid (>50%) SLEDAI-2K disease activity score reduction within days after three NucleoCapture® treatment sessions without escalating immunosuppression, thus supporting fast-acting immunomodulation by NucleoCapture® .
Santersus is preparing a pivotal, multinational, multicentre, randomised, parallel-group, open‑label, efficacy and safety clinical trial of NucleoCapture® in patients with active moderate to severe SLE. The study is due to start patient recruitment in QI2026.