Clinical Applications: Sepsis
NucleoCapture® for Sepsis
NucleoCapture® mode of action
Neutrophil Extracellular Traps (NETs) and other chromatin associated molecular patterns are newly characterized class of early super alarmins reflecting the totality of cell death products released upon occurrence of NETosis , apoptosis, necrosis, pyroptosis, and necroptosis in septic patients.
Release of NETs and other chromatin associated molecular patterns (CAMPs) in sepsis triggers vascular leak, microvascular obstruction, metabolic changes, gut bacterial translocation, immunosuppression and uncontrolled inflammation through direct activation of plethora of signaling pathways: TLR2 TLR4 , TLR3 , TLR7 , TLR8 , TLR9 , AIM2 , IFI16 , cGAS /STING, RAGE , NLRP3, RIG1 , MDA5 , TREM-1. The culmination of these events can lead to multiple organ failure, and ultimately, death.
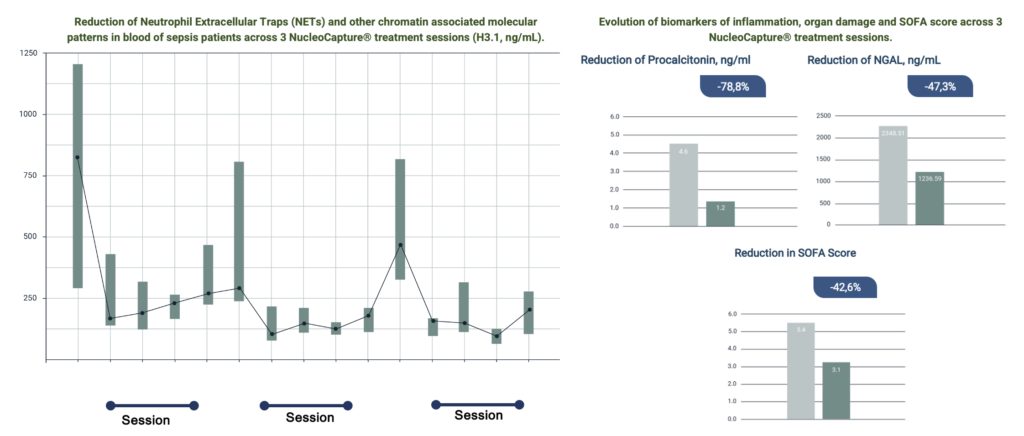
NucleoCapture® therapeutic apheresis session results in a rapid and sustained reduction of concentration NETs and other chromatin associated molecular patterns in circulation thereby attenuating vascular and organ disfunction, inflammation and immunosuppression.
Preclinical development
A randomized blinded sham-controlled study of NucleoCapture® in porcine septic shock model involving a critical care protocol showed that NucleoCapture® effectively removes NETs and other CAMPs from circulation, reduces inflammation and significantly improves metabolic state, haemodynamics and organ function leading to resolution of septic shock and improved survival.
In January 2022, the U.S. FDA granted Breakthrough Device Designation for NucleoCapture® for the treatment of sepsis recognizing the urgent medical need and first-in-class novel promising mechanism of action.
Clinical development
Santersus has completed its first in human exploratory clinical trial of NucleoCapture® in patients critically ill with sepsis.
NucleoCapture® treatment stabilized and improved outcomes for critically ill patients who otherwise were expected to die rapidly. This was associated with reduction in NETs, inflammatory markers, organ damage, and decreased need for vasopressors and organ support. Across more than 40 treatment sessions performed, NucleoCapture® was well tolerated with no adverse events. It was safe and easy to use NucleoCapture® in the ICU environment.
Santersus is preparing a pivotal, multinational, multicentre, randomised, parallel-group, adaptive, open‑label, efficacy and safety clinical trial of NucleoCapture® in patients with sepsis due to pneumonia. The study is due to start patient recruitment in QI2026.